Available cartridges and reactions
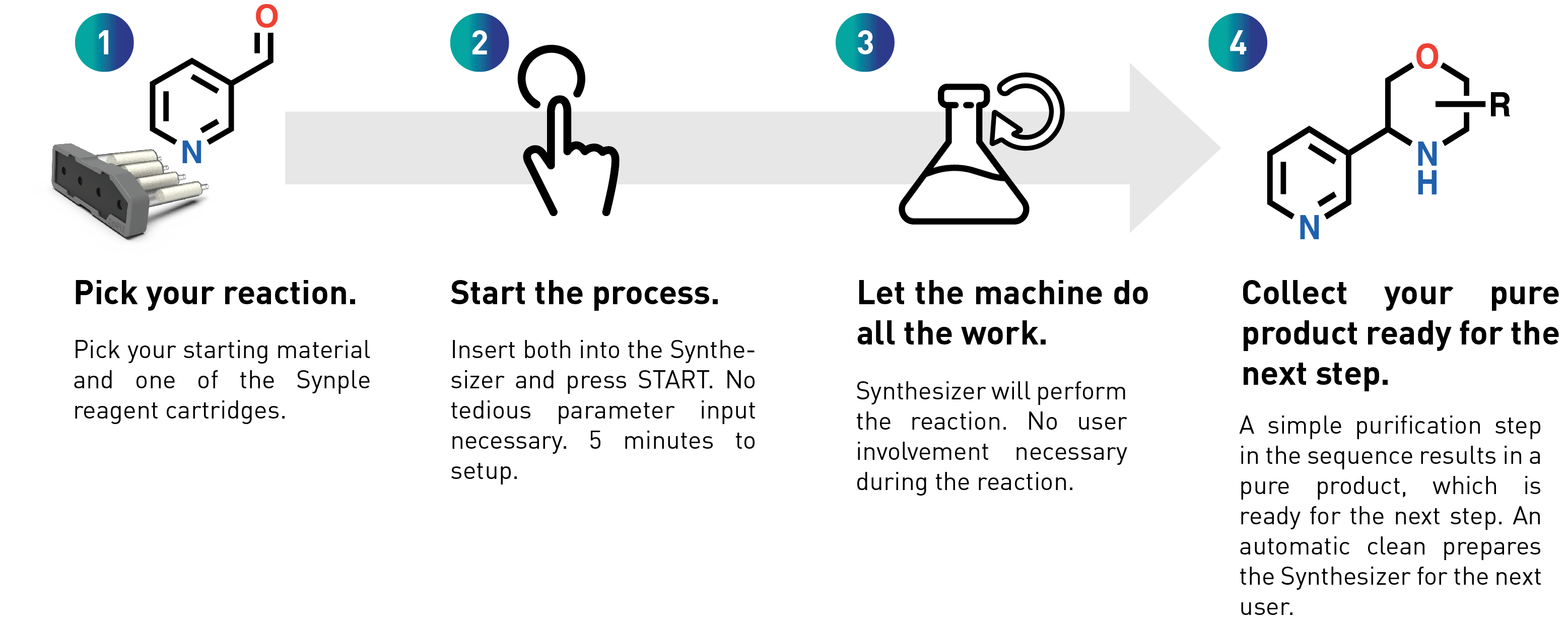
How does Synple work?
Settings up a reaction on Synple 2
Comparison of Synple 1 vs manual synthesis
Available reaction classes
N-Heterocycle formation

- 10 different Heterocycle structures currently available
- Scale: up to 0.5 mmol
Advantages compared to classical batch chemistry are:
- Minimise user exposure to toxic tin reagents
- Multistep reactions completed at the touch of a button
- High reproducibility
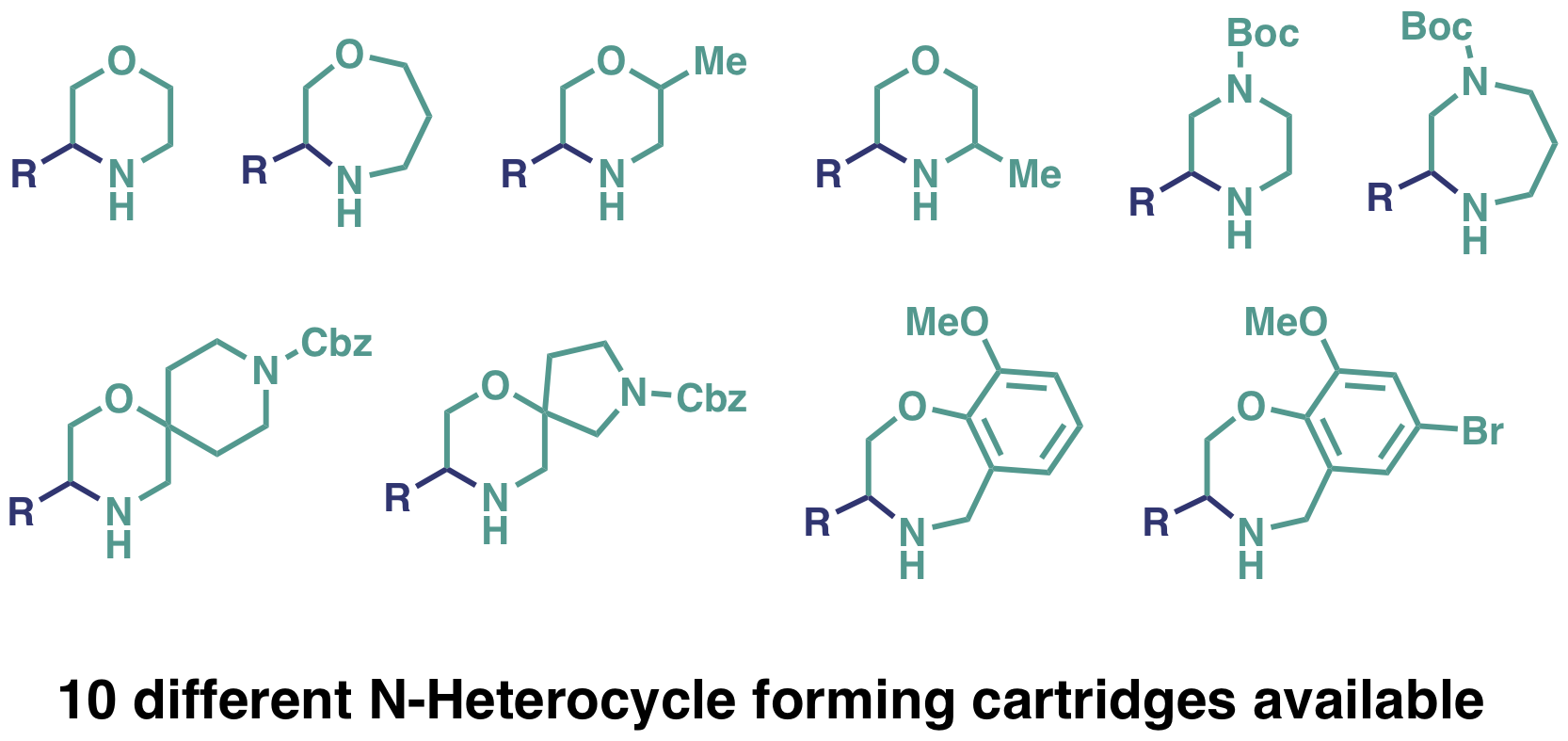
What is in the heterocycle formation cartridges:

Reductive Amination
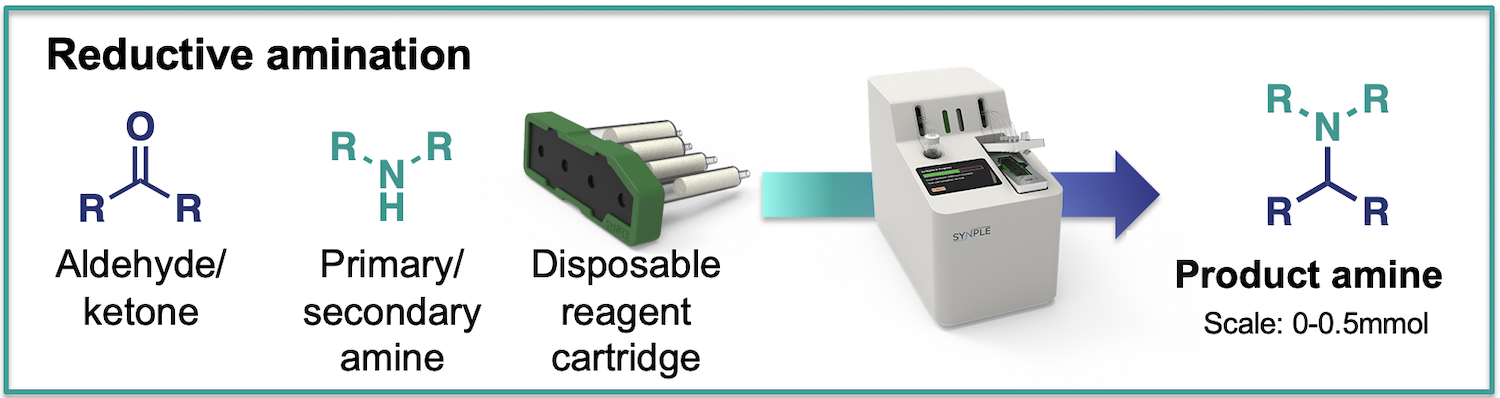
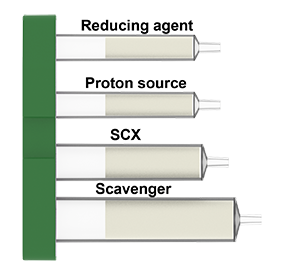
The cartridge contains all reagent for the condensation, reduction and purification steps of the process.
- Scale: up to 0.5 mmol
- Time-saving – reaction, work up and purification in a single process
- Automation of routine chemistry to allow more time for technically demanding reactions
- Generic methods, selected based on reaction partners, minimize need for reaction optimization.
What is in the reductive amination cartridges:
Mitsunobu Reaction
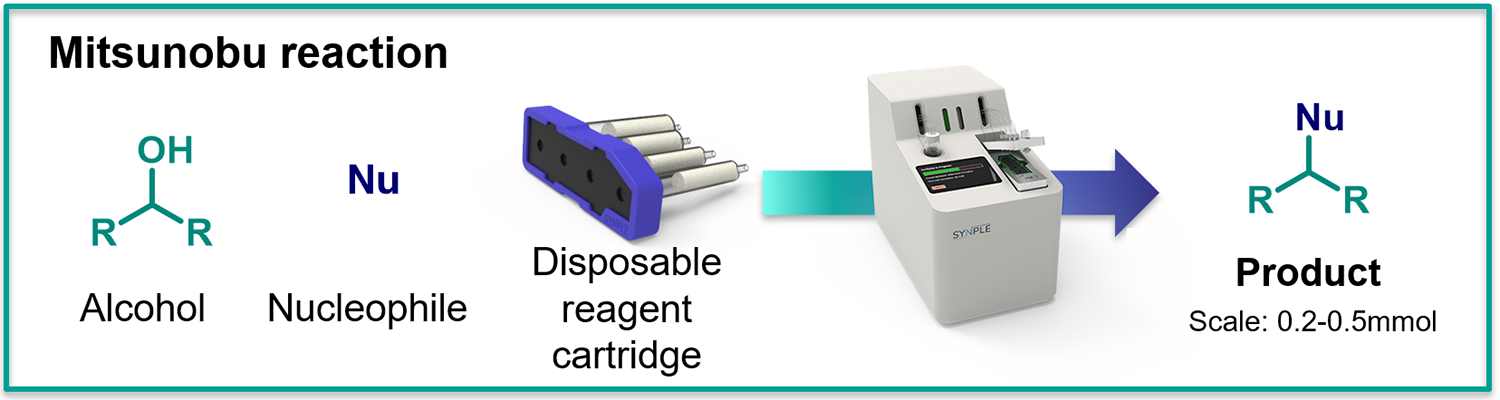
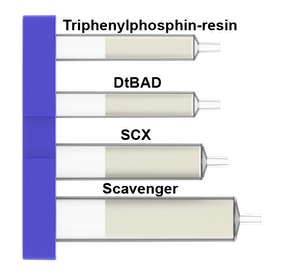
- Scale: 0.2 to 0.5 mmol
- Applicable to a range of pronucleophiles (phenols, phthalimides, tosylamides, tosylhydrazones and carboxylic acids).
- Only 5-10 setup time is required to obtain Mitsunobu product.
- No tedious removal PPh3O necessary.
What is in the Mitsunobu cartridges:
PROTAC formation

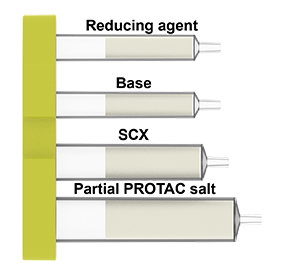
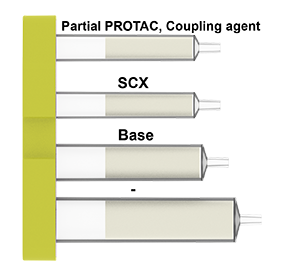
The cartridge contains all reagents for the linking the carbonyl group of a protein binder with the partial PROTAC and components for the purification steps of the process.
- Scale: up to 0.1 mmol
- Coupling of amines, carboxylic acids, ketones and aldehydes
- Cartridges available for coupling via reductive amination and amide formation
PROTAC cartridge content:
Via reductive amination
Via amide formation
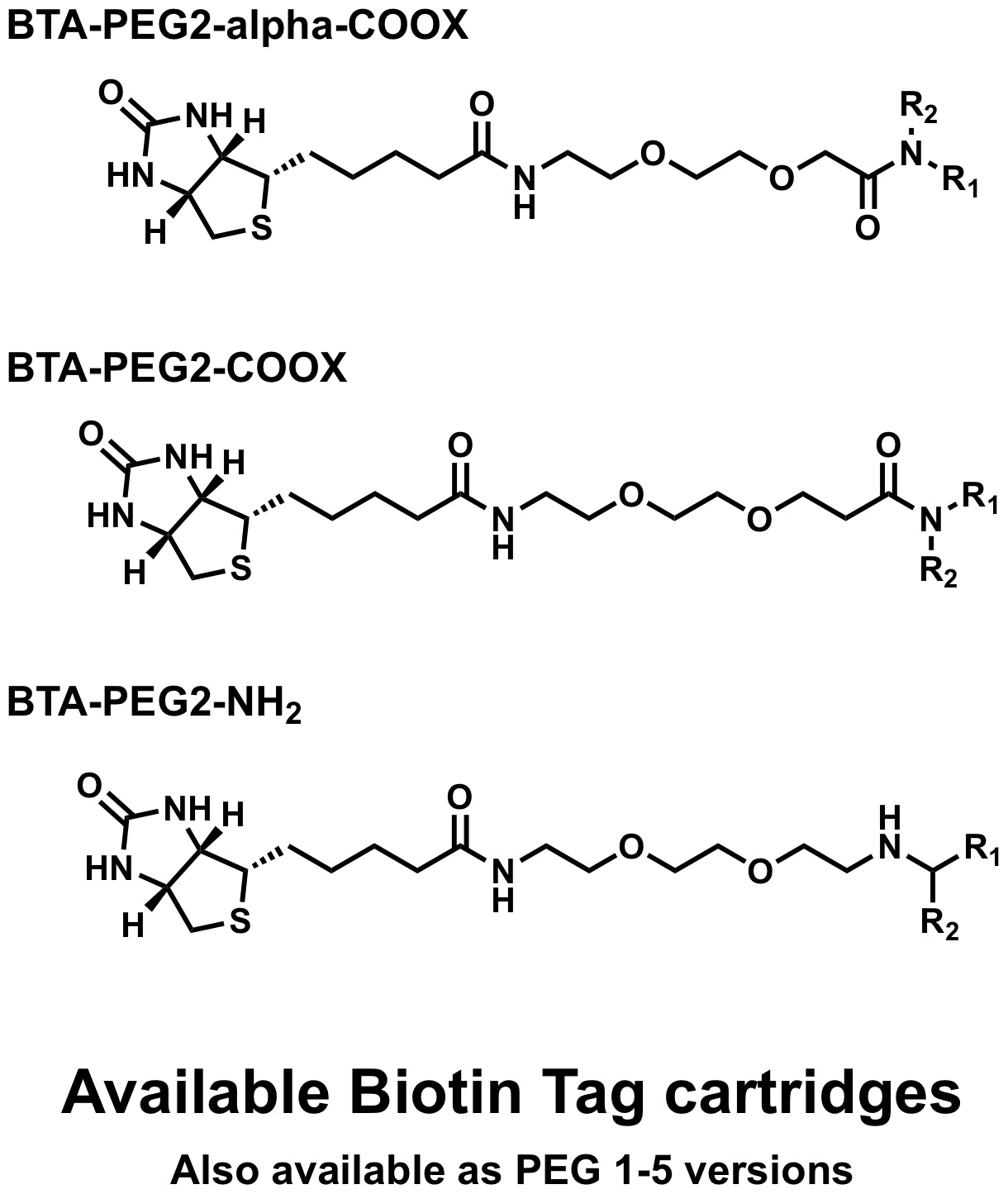
Biotin Tags
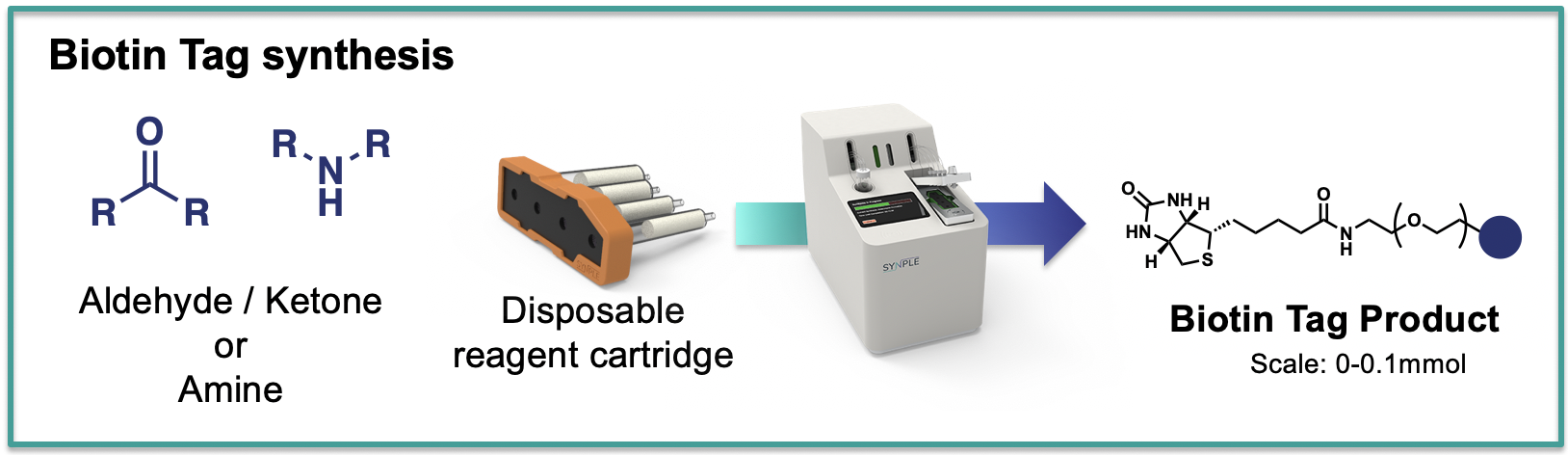
Biotin Tags
The cartridge contains all reagents for the linking the compound of choise to a variety of biotin tags and also includes components for a simple purification step.
- Scale: up to 0.1 mmol
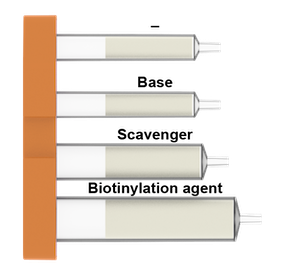
What is in the Biotin cartridges:
For Amine-Linker
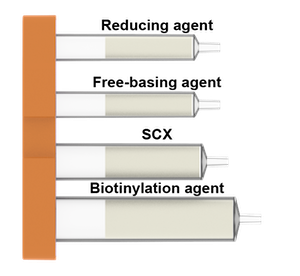
For Amide-linker
Deoxyfluorination

Deoxyfluorination
Fluorinations are among the most interesting reaction in medicinal chemistry and mostly used for late stage functionalization.
The subgroup of Deoxyfluorination involves the reaction between an alcohol and a fluorinating agent to generate the corresponding fluorinated product.
Using the approach the Synple Chem synthesizer offers an easy and fast automated method for the deoxyfluorination of primary and secondary alcohols without the need of handling fluorinating agents.
- Scale: up to 0.2 mmol
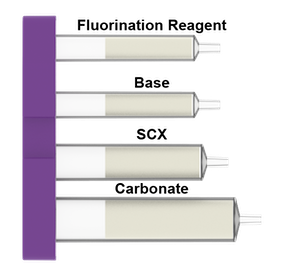
What is in the Fluorination cartridges:
Boc Protection

Boc protection
- Scale: two sizes available; up to 0.5 mmol and up to 1.2 mmol
What is in the Boc protection cartridges:
Boc Deprotection

Boc deprotection
N-Boc deprotection involves the reaction between an N-Boc protected amine and acid (TFA, HCl, TsOH and etc…) to generate the corresponding free amine salt.
Using the approach in this application note, the Synple Chem synthesizer offers an easy and quick automated method for the N-Boc deprotection of primary and secondary amines and avoid the handling of volatile and corrosive acids such as TFA or HCl.
- Scale: up to 0.5 mmol
- Avoid handling volatile and corrosive acids
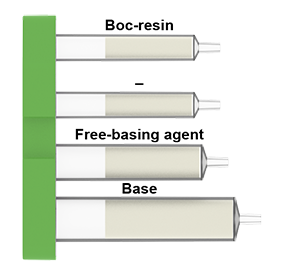
What is in the Boc deprotection cartridges:
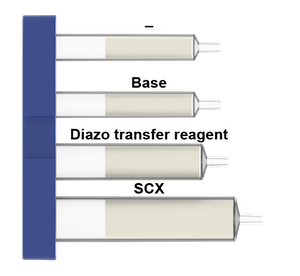
Azide formation

Azide formation
Azide formation by diazo transfer of primary amines is a highly important reaction in organic chemistry and chemical biology. Azides have been widely utilized as the key partner in Cu-catalyzed azide-alkyne cycloaddition reactions for bioorthogonal click chemistry, peptide conjugation and polymerization processes. Azides also show good chemical stability so that they can serve as a protecting group for primary amines, in particular in the field of carbohydrate chemistry. Previous methods of converting amines into azides require fresh preparation of diazo transfer agents (not commercially available) using highly hazardous reagents, which has largely limited its broad adoption.
- Scale: up to 0.5 mmol
- Avoid handling azidation reagents
- Two version available for alkyl amines A001/A101 and alkyl amines A002/102
What is in the Azide cartridges:
Amide formation
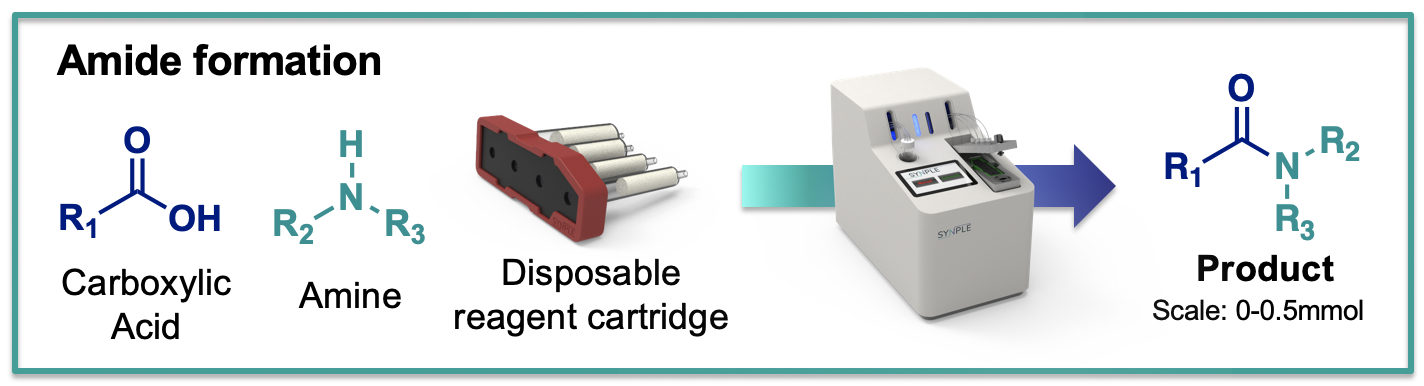
Amide formation
The amide (carboxamide) group is one of the most important functionalities broadly occurring in small molecules, peptides, proteins and various natural/synthetic polymers. In pharmaceutical industry, amide bond formation covers nearly 25% of the patent reactions, thus presents as the most widely used synthetic method in drug discovery. Amide formation between a carboxylic acid and an amine initated with the pre-activation of the carboxylic acid with a coupling agent to form an active intermediate, such as acyl halides, acid anhydrides and active esters, which undergoes a nucleophilic replacement of the amine to form the amide product. Numerous coupling agents have been developed and become readily available from commercial vendors. Recently, increased concerns over these coupling agents as skin sensitizers prompt research in finding safer alternatives and/or improving existing protocols to meet the upgraded laboratory standards. Using a solid-supported coupling agent for the amide formation has turned into a suitable and welcoming solution in both bath and flow setup for minimizing the exposure of sensitizing chemicals and simplifying the purification of the amide product.
- Scale: up to 0.5 mmol
What is in the Amide cartridges:
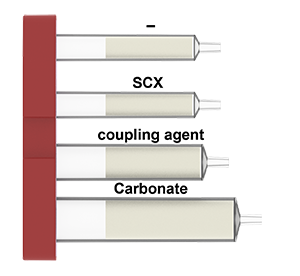
Silyl Deprotection

Silyl deprotection
Silyl group deprotection involves the reaction between a silyl protected alcohol and an acid (TFA, HCl, CSA and etc.) or a fluoride (TBAF, HF pyridine and etc.) to generate the corresponding free alcohol. This transformation is a widely used reaction in organic chemistry.
Using the approach in this application note, the Synple Chem synthesizer offers an easy and quick automated method for the silyl deprotection of primary and secondary alcohols and avoid the handling of volatile and corrosive acids such as TFA or HCl or fluorine source as TBAF.
- Scale: up to 0.5 mmol
- Avoid handling volatile and corrosive acids
- Available for us with free amines (A011/A111) and amine salts (A012/A112)
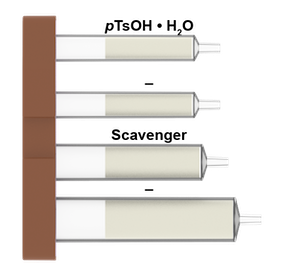
What is in the Silyl deprotection cartridges:
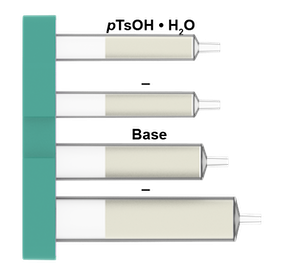
Suzuki coupling
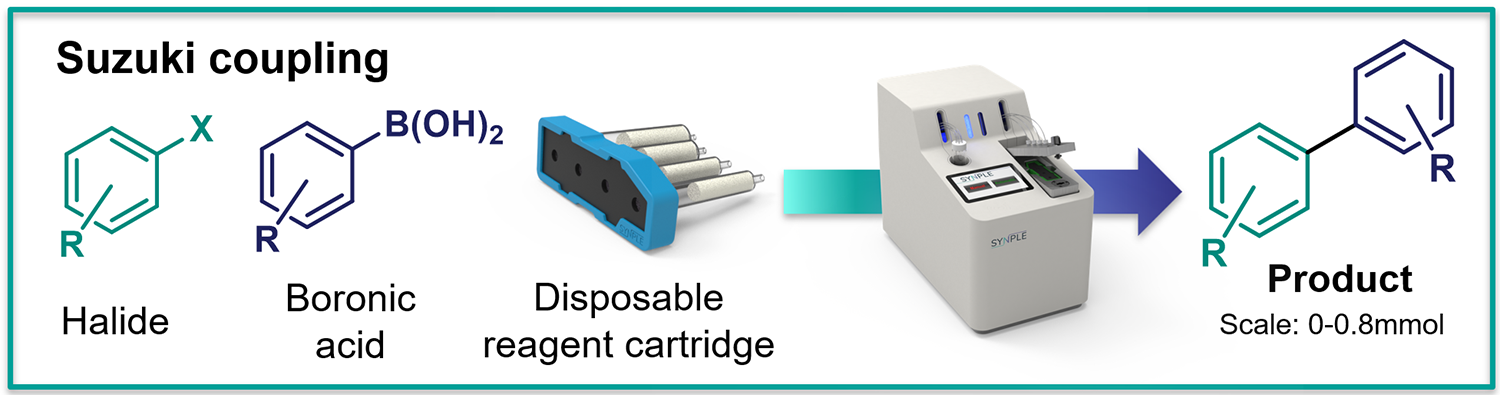
Suzuki coupling
- Scale: up to 0.8 mmol
- Coupling aryl bromides (aryl chlorides also possible) with aryl boronic acids
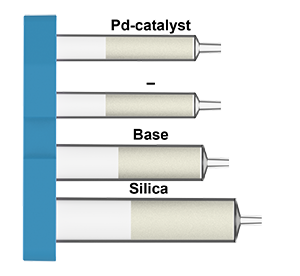
What is in the Suzuki coupling cartridges:
Cbz protection

Cbz protection
Nitrogen, as a key element with existence in diverse forms, participates in various metabolic and physiological functions in the living organisms. As the most important N-centered functional group, amino group presents widely in small molecules, peptides and proteins. Due to its nucleophilic nature, amino group often requires protection in the multistep synthesis as a means to block reactive sites with close proximity and sometimes induce more favorable solubility for reaction handling and product purification. Many protecting groups for amino group have been developed in the past century and they have become a set of must-have tools for all phases in research and development. Carbamates are an important class of protecting groups, such as tert-butyl carbamate (Boc), benzyl carbamate (Cbz) and 9-fluorenylmethyl carbamate (Fmoc). Among them, Cbz is one of the most broadly used for, 1) its stability against many acidic, basic or oxidative conditions and inert toward many electrophilic reagents; 2) both installation (with Cbz-Cl) and removal (under reductive conditions) are relatively straightforward.
Though Cbz-Cl (benzyl chloroformate) is easily used for N-Cbz protection, it is a highly toxic reagent which may induce cancer. Cbz-Cl is also a lachrymator with an acrid odor, therefore its handling may pose certain safety issue in the laboratory. Usually N-Cbz protection reaction requires a basic aqueous workup to remove the excess amount of Cbz-Cl, which is tedious and less ideal. To avoid the usage of Cbz-Cl, Cbz-OSu (N-(benzyloxycarbonyloxy)succinimide) represents as a non-toxic alternative. By using pre-packed Cbz-OSu cartridge and solid-phase purification method, N-CBz protection has become safer and more user-friendly.
Using the approach in this application note, the Synple Chem synthesizer offers an easy and fast automated method for the N-Cbz protection of primary and secondary amines and amine salts.
- Scale: up to 0.8 mmol
- Avoid handling volatile and corrosive acids
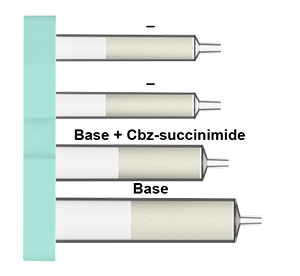
What is in the Cbz protection cartridges:
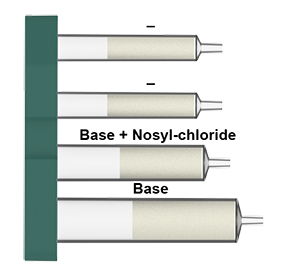
Nosyl protection

Nosyl protection
Nitrogen, as a key element with existence in diverse forms, participates in various metabolic and physiological functions in the living organisms. As the most important N-centered functional group, amino group presents widely in small molecules, peptides and proteins. Due to its nucleophilic nature, amino group often requires protection in the multistep synthesis as a means to block reactive sites with close proximity and sometimes induce more favorable solubility for reaction handling and product purification. Many protecting groups for amino group have been developed in the past century and they have become a set of must-have tools for all phases in research and development. Carbamates are an important class of protecting groups, such as tert-butyl carbamate (Boc), benzyl carbamate (Cbz) and 9-fluorenylmethyl carbamate (Fmoc). As an alternative to carbamates, it was established that using nitrobenzenesulfonamides (Ns) is an efficient and versatile synthetic method for the protection of amines. This class of protecting group represent an interesting class because of, 1) its stability against many acidic, basic conditions; 2) both installation (with Ns-Cl) and removal (under mild conditions) are relatively straightforward; 3) its role as an activating group for the synthesis of secondary amines from primary amines.
Though o-Ns-Cl (2-Nitrobenzenesulfonyl chloride) is easily used for N-o-Ns protection, it is a corrosive reagent, which may cause severe skin burns and eye damage. Usually N-o-Ns protection reaction requires a basic aqueous workup to remove the excess amount of o-Ns-Cl, which is tedious and less ideal. By using pre-packed o-Ns-Cl cartridge and solid-phase purification method, o-Ns protection has become safer and more user-friendly.
Using the approach in this application note, the Synple Chem synthesizer offers an easy and fast automated method for the o-Ns protection of primary and secondary amines and amine salts.
- Scale: up to 0.8 mmol
- Avoid handling volatile and corrosive acids
What is in the Nosyl protection cartridges: